Lithium-Ion Batteries: The 18650
Chris Greene
The Micro-mobility or E-mobility industry has been receiving a lot of publicity over the past few years—and for all the wrong reasons. Through the media, online videos, and direct experience, we have all been witness to the increasing frequency of fires involving E-bikes/scooters. This phenomenon has drawn a lot of attention to this industry, and deservedly so, but it is important to understand the underlying energy source—the 18650 lithium-ion cell. It is the workhorse of the E-mobility, power tool, and portable power supply industry and one of the most common lithium-ion cells on the market. It is small enough to power an E-cigarette/vape cartridge, easily fitting into your pocket, or can be strung together to power much larger items that have become commonplace in the American home.
This cylindrical battery, which derives its name from its dimension—18mm diameter by 65mm in length—was developed in 1994 in response to an increasing demand for smaller and more powerful portable battery-operated devices. The 18650 cell offered a small/compact lightweight rechargeable energy source with high battery voltage—3.2v to 4.2v. The battery had an added benefit of being modular, making it appealing to a variety of rechargeable handheld products as well as larger stationary storage uses.
The 18650 is often compared to an AA battery because of the cylindrical similarities, but this is where the similarities end. The 18650 is larger in size than the AA battery, and its voltage is more than double. The energy density is four-plus times greater than that of an AA battery, but the real difference is in how long this battery can power a device and how fast it can offload its power when needed. This combination of longer-duration use and high cycle life coupled with its ability to offer high-output demands is rare. The 18650 fits into a category of batteries often described as “high drain” and is suited to portable devices that required constant higher voltage such as E-cigarettes, laptop computers, and power tools.
How It Works
Like all rechargeable batteries, it has three primary components: an anode, a cathode, and a separator. The design allows electrons to flow in both directions—hence, the “rechargeable capability.” This is different from a standard traditional nonrechargeable battery where electrons flow in one direction until the battery is depleted or “dead.” Rechargeable batteries are more complicated than your standard battery, and manufacturers employ a variety of proprietary chemistries to achieve their goals.
Unique to the lithium-ion battery is the use of lithium-ion particles in a liquid electrolyte suspension medium. This battery is designed to allow electrons to move from anode to cathode through the “load” as ions flow through the separator. The separator serves an important role and offers some insight as to how these batteries may fail. It keeps the anode and cathode separate; however, it needs to allow ions to flow back and forth through it during the discharge and charging cycle.
When the separator fails, the anode and cathode can contact each other and short out, creating a spark inside the battery. The electrolyte solution for the lithium particles is extremely flammable. As the separator fails and the anode and cathode fault, heat is generated and gas forms inside the battery cell. As the heat and pressure build, the battery casing weakens and fails, resulting in gas discharge from the cell. Some failures occur through the vent port, while others cause swelling of the casing, leading to a breach. This failure process can take days or happen instantly.
The failure of one 18650 cell may seem harmless, but consider this: One 18650 lithium-ion battery can produce eight liters of toxic/flammable gas. Many devices use several 18650 cells to work. In the case of a typical electric bike/scooter, the battery may contain between 50 and 130 cells. That’s a lot of heat and a lot of gas. Complicating this battery failure process is that hydrogen gas is one of the primary gases produced when a lithium-ion battery fails. So, we now have a combination of high heat, a spark leading to fire, and a gas compound that includes a high concentration of hydrogen gas discharging at high pressure like a mini blowtorch.
Figure 1. Common Gases Produced from Lithium-Ion Device Failure
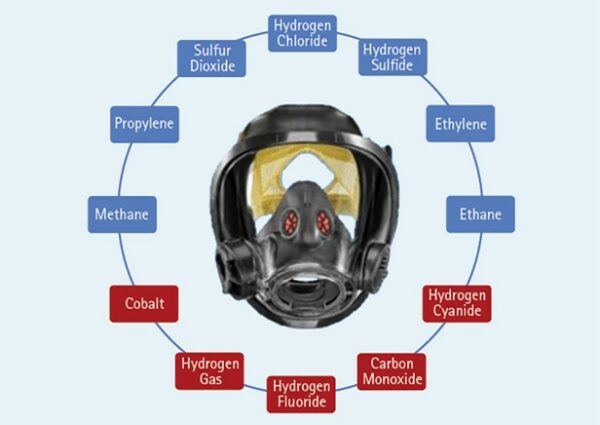
This situation is not unique to the 18650 cell; it is the basic failure domino effect for any lithium-ion rechargeable battery format. This article focuses on the 18650 because of its total market saturation in the mobile and stationary energy storage space but, most importantly, because of how common this cell is in our homes. If you own a rechargeable drill that is less than 10 years old, it is likely powered by 18650 cells. That new 20v power pack for your cordless drill is five 18650 3.7v cells wired in series. If you have a 40v, then you have 10 18650s powering that tool.
An electric scooter may have as many as 130 18650 cells inside its power pack battery. If that battery fails catastrophically, that’s a lot of gas. If you own an E-mobility device, it is 99.9% powered by the 18650 cells. When considering a fire scenario involving this device, it’s a simple question of how many 18650 cells are in the battery pack and the location of the bike in your home.
18650 Cell Chemistry
The design chemistry for these cells is numerous. The most common ones in use are nickel cobalt aluminum (NCA), nickel manganese cobalt (NMC), and lithium iron phosphate (LiFePO4). Each chemistry has a specific strength of application.
NMC is currently the most popular chemistry, with a sizeable market share. It is cheap, has a good cycle life, and is considered a durable battery. This is likely what you see in most E-bikes, power tools, and some electric vehicles.
NCA is most noted for its superior power density and low passive discharge rate (a term used to describe a battery cell’s propensity to lose power while not in use). This battery chemistry has been a staple for Tesla vehicles—some models have more than 7,000 18650 cells.
LiFePO4 is considered the most thermally stable of the three chemistries; however, it has a lower energy density by volume, which means more cells may be needed to achieve the same energy density as an NCA. With stationary battery energy storage systems (BESS) facilities, where space is not necessarily an issue, this chemistry has become quite popular. One of the hazard considerations for this chemistry is that when LiFePO4 cells fail, the off-gas contains more hydrogen gas by volume that any of the other lithium-ion battery chemistries. Couple this with an enclosed BESS facility, with typical capacity in excess of 10 megawatts, and the hazard profile goes up tremendously. A BESS exploded in Surprise, Arizona, in April 2019 and injured eight firefighters and one police officer. That was a two-megawatt BESS of LiFePO4 cell chemistry. For the Seattle (WA) Fire Department’s Energy Response Teams, any fixed-facility BESS involved in a fire situation mandates an automatic “Defensive Posture” from the onset. The liquid electrolyte solution acts as a suspension medium for the lithium-ion particles. The electrolyte is extremely flammable.
Fire Gas Considerations
Most of the fire gases will be similar to those found in a couch/car fire, but there are a few new ones that we have not seen at this scale before.
Hydrogen gas is one of the primary fire gases present with any incident that involves lithium-ion batteries and creates thermal and explosive issues. We must be prepared for this eventuality. Whether the batteries are the cause of the fire or are damaged as a result of proximity to the fire matters not; the result will be the same: Up to 40% of the fire gases produced by volume can be hydrogen gas.
This is new. We have not seen hydrogen gas in residential spaces prior to this—not to this scale, anyway. This change is a result of two factors: First, all lithium-ion batteries will produce hydrogen gas when they fail. Each chemistry may produce a different amount of hydrogen gas, but it will be present. Second, this is a ubiquitous energy source. From the handheld E-cigarette to computers to the E-mobility, all are powered by this technology. As you can see, these devices are in every home in America, and that is not going to change anytime soon.
For operations, ensure continuous ventilation and don’t leave one single lithium-ion cell behind. This means increased time on scene performing a thorough overhaul. Don’t let the thermal imaging camera (TIC) dictate whether a lithium-ion cell represents a hazard. That cell may read cold on the TIC, but that does not mean that the cell is not in the slow process of failing. Get every cell out of the building. Cells left behind at fires that were believed to be fully extinguished have rekindled days later. The overhaul game has changed, so take your time and be thorough. Remember, no cells left behind.
Hydrogen fluoride gas. Like hydrogen gas, we have not seen hydrogen fluoride gas to this scale in traditional firefighting spaces. Beyond wearing your full personal protective equipment (PPE) with self-contained breathing apparatus (SCBA), it is going to be difficult to safeguard for this gas when lithium ion is present. Like hydrogen gas, hydrogen fluoride gas is invisible. It may be present even when a fire-damaged product is reading cold on your TIC. Stay vigilant. Wear your SCBA and ventilate continuously until all cells have been removed from the structure. And, of course, launder 100% of your PPE immediately as you would after any fire.
The 18650 lithium-ion cell is one of the most common building blocks for battery designers. These products will be with us for a long time, and we must understand the hazards and that the fundamental firefighting tactics in the residential space may need to change.
